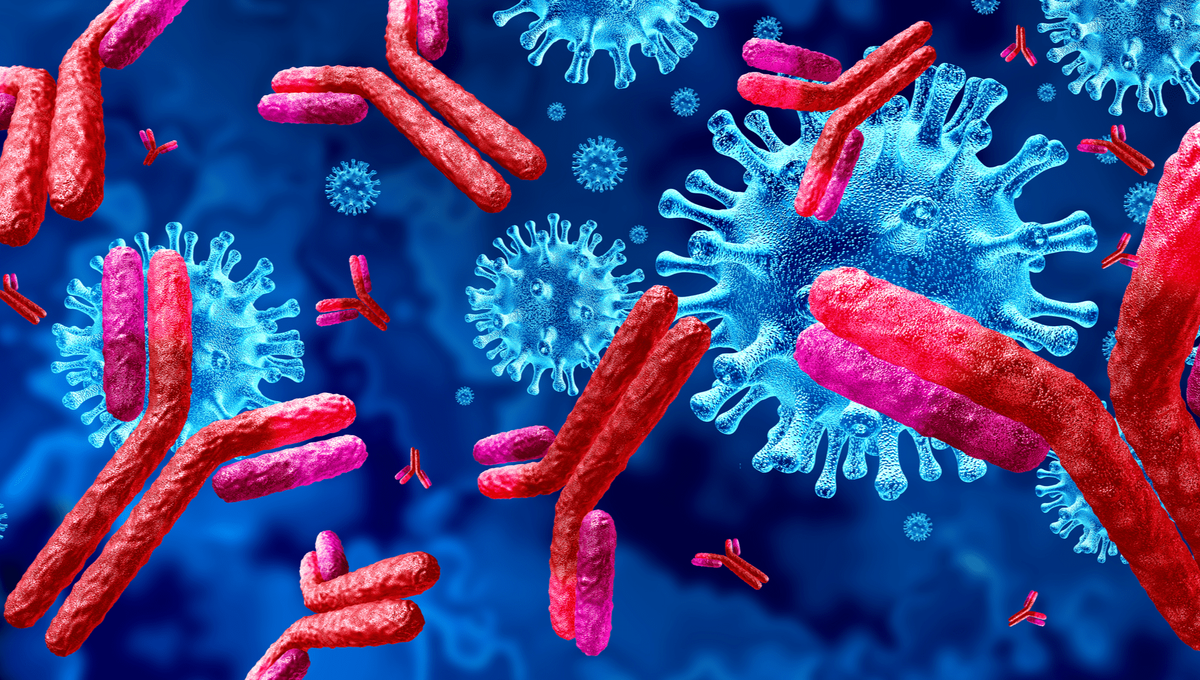
Antibodies, also known as immunoglobulins, are Y-shaped proteins produced by B cells of the immune system that help identify and neutralize pathogens like bacteria and viruses. By binding to specific molecules, known as antigens, on the surface of pathogens, antibodies help eliminate them from the body and prevent future infections. Their structure enables them to recognize an almost limitless array of pathogens while also retaining the flexibility to evolve and recognize new threats. In this article, we explore the remarkable properties and functions of these essential immune proteins.
The Structure of Antibodies
Antibodies are composed of basic structural units known as immunoglobulin. Each immunoglobulin unit contains two identical heavy chains and two identical light chains bound together by disulfide bonds. The light chains are attached to the fragment antigen-binding or Fab region which contains the antigen-binding sites. The stems of the Y-shaped structure are formed by the heavy chains. At the end of each arm of the Y are antigen-binding sites with an incredible ability to recognize different shapes on antigens. The stem region is constant while the Fab region is variable, allowing antibodies to bind to an immense range of pathogen structures.
Classes of Antibodies
There are five major classes or isotypes of Antibodies in mammals: IgG, IgA, IgM, IgD, and IgE. IgG is the most abundant antibody found in blood and extracellular fluid. It effectively activates various immune cells to clear pathogens. IgA predominates in secretions like tears, saliva, and breast milk, providing immune protection at body surfaces. IgM is usually the first antibody produced during initial infections. It activates the complement system to destroy pathogens before they spread. IgD and IgE activate mast cells and basophils during allergic reactions. Each class has adapted specialized roles defending the body against different types of threats.
Generating Diversity
A critical component of the adaptive immune system is its ability to generate antibodies with millions of different antigen binding specificities. This diversity arises from genetic recombination events that occur during B cell development in the bone marrow. Segments of DNA that encode the variable regions of the heavy and light chains undergo DNA rearrangements, forming new combinations of heavy and light chain gene segments. Additional diversity comes from junctional diversity created during joining of the gene segments and random additions or deletions of nucleotides. Furthermore, somatic hypermutation introduces mutations during affinity maturation, enabling antibodies to evolve and tightly bind emerging pathogens. Through these mechanisms, the antibody repertoire can react to virtually any molecular structure found in antigens, providing immunity against millions of potential pathogens.
Mechanisms of Action
Once antibodies bind pathogens by recognizing antigenic epitopes, they neutralize them through various mechanisms of action. Opsonization tags pathogens to be phagocytosed by immune cells like macrophages and neutrophils and may even trigger killing by complements, pore forming proteins that disrupt pathogen membranes. Agglutination crosslinks antigens and causes clustering of pathogens to immobilize them. Precipitation effectively removes soluble antigens from tissues by forming large antigen-antibody complexes. Cytotoxicity involves antibody-dependent cell-mediated cytotoxicity (ADCC) where an antibody binds both an antigen on a pathogen along with receptors on immune cells like natural killer cells, causing the immune cells to destroy the pathogen through release of toxic granules or cytokines. Antibodies are also instrumental in providing long-term immunity through immunological memory by activating B cell memory and generating long-lived plasma cells after resolution of infection or disease.
Monoclonal Antibodies
The precise antigen-binding specificities of monoclonal antibodies make them powerful tools for medical research as well as treatment of diseases. In 1975, Köhler and Milstein developed the first method of creating monoclonal antibodies in the laboratory by fusing B cells with myeloma cancer cells, enabling mass production of identical antibodies with a single specificity. Since then, monoclonal antibodies have revolutionized biomedical research by serving as invaluable detection reagents for antigens and proteins. Clinically, they are used to treat autoimmune disorders, cancer, transplant rejection and many infections by targeting pathogenic antigens without impacting the rest of the immune system. Areas of active research include engineering monoclonal antibodies for better targeting abilities and reduced side effects as well as development of bispecific antibodies simultaneously binding two antigens. The future of therapeutics holds much promise in utilizing monoclonal antibodies for personalized treatment of diverse medical conditions.
Applications of Antibody Technology
Antibodies form the basis of important diagnostic tests due to their specificity for antigens. ELISAs or enzyme-linked immunosorbent assays use enzymatically labeled antibodies to detect disease antigens or autoantibodies. Immunohistochemistry and immunofluorescence utilize fluorescently labeled antibodies to visualize cellular antigens as microscopically detectable stains. Rapid lateral flow tests use antibody-antigen reactions to deduce qualitative results outside clinical lab settings. In research, immunoprecipitation and western blotting employ antibodies to pull down and identify proteins of interest. Phage display technologies apply antibodies for affinity selection and generate antibody libraries. Mass spectrometry relies on antibodies to capture, enrich, and analyze low abundance proteins or modifications. Overall, antibodies have revolutionized our understanding of biology and mechanisms of disease through their diverse array of applications as invaluable detection molecules.
In summary, antibodies are key effector molecules of the adaptive immune system essential for protection against pathogens. Their ability to bind a virtually unlimited repertoire of antigens with high specificity and affinity arises from elegant molecular structures and exquisite mechanisms of genetic recombination and somatic mutation. By tagging pathogens, activating immune functions, and neutralizing toxic molecules, antibodies clear infections and prevent recurrence of diseases. Moreover, monoclonal antibody technologies have proven transformative for research, diagnostics and targeted therapies. The full scope of antibody functions and applications continues to uncover surprising insights, cementing these powerful yet flexible immune proteins as central players in human immunity and biomedicine.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it