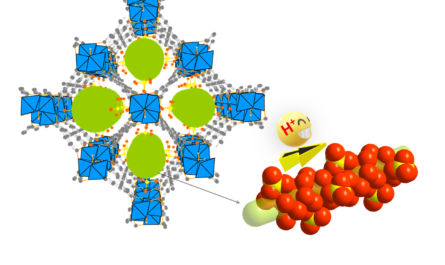
In a significant breakthrough for chemistry, researchers from the Karlsruhe Institute of Technology (KIT) have developed a system for the activation and catalytic transfer of ammonia. This newly developed process offers a sustainable source of nitrogen and has the potential to revolutionize the production of amines, which are highly sought-after as building blocks in various industries.
Ammonia (NH3) is a compound consisting of nitrogen and hydrogen, and it is widely used in the production of a range of nitrogen-containing chemicals. Amines, which are organic derivatives of ammonia, are particularly in high demand as key components for agricultural and pharmaceutical chemicals, detergent substances, dyes, lubricants, coatings, and catalysts in the production of polyurethanes. Ammonia is also used in gas scrubbers at refineries and power plants.
Traditionally, the production of amines from ammonia and unsaturated hydrocarbons, known as hydroamination, has been a challenge due to the lack of a suitable catalyst. Conventional catalysts based on transition metals are reactive with ammonia and become ineffective, making the process inefficient and wasteful. Finding a sustainable and efficient method for hydroamination has been a long-standing goal in the field of catalysis.
The researchers at KIT, in collaboration with researchers from Paderborn University and Complutense University of Madrid, have developed a system for activating ammonia that is not based on transition metals but instead uses main group elements. This innovation offers an “atom-economic” process that does not produce any waste, making it highly sustainable.
The team created a frustrated Lewis pair (FLP), consisting of an acid as an electron pair acceptor and a base as an electron pair donor. Normally, these two components react to form an adduct. By preventing or limiting the formation of the adduct, the molecule becomes “frustrated” and readily reacts with small molecules such as ammonia.
The key to the success of this system is to control the reactivity of the FLP to ensure that the reaction with small molecules is reversible. This breakthrough allowed the researchers to split the nitrogen-hydrogen bond of ammonia at room temperature, opening the door for NH3 transfer reactions catalyzed by a catalyst based on main group elements.
While the team has currently only converted activated substrates and not unsaturated hydrocarbons, they believe they are well on their way to achieving their ultimate goal. The researchers anticipate that this proof of concept will stimulate further research into using N-H-activated ammonia as an easily accessible and sustainable source of nitrogen.
This discovery has significant implications for various industries, as it could lead to a more sustainable and efficient method for producing amines. By utilizing ammonia as a readily available source of nitrogen, the need for transitioning metals and the resulting waste can be greatly reduced. This breakthrough offers a promising solution for meeting the high demand for amines while minimizing environmental impact. Further research and development in this area could transform the chemistry industry and contribute to a more sustainable future.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it