Introduction
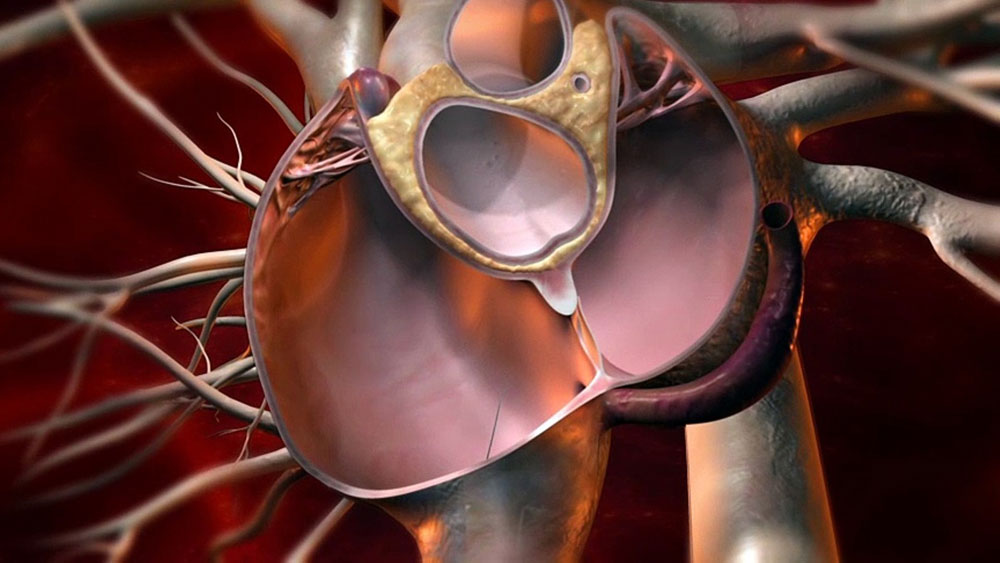
A patent foramen ovale (PFO), or patent ductus arteriosus, is a physical condition present since birth that allows blood flow between the left and right upper chambers of the heart (atria). While PFO itself is very common and usually harmless, it has been linked to an increased risk of stroke or migraine headaches in certain patients. There are medical devices available known as PFO closure devices that can close a PFO through a minimally invasive cardiac catheterization procedure, aiming to reduce the risk of recurrent strokes or migraines. This article will discuss PFO closure devices in more detail.
Types of PFO Closure Devices
Two major types of PFO closure devices that are commonly used are:
– CardioSEAL Septal Occluder: This was one of the first generation PFO closure devices approved by the FDA. It consists of a nitinol mesh “umbrella” covered in polyester fabric on one side. An delivery cable is used to place the occluder through a catheter across the PFO, where it expands to close the opening from the left atrium.
– Amplatzer PFO Occluder: This has become the most widely used PFO closure device. It consists of two self-expanding nitinol discs linked by a short connecting waist. Similar to the CardioSEAL device, it is delivered via catheter and expands across the PFO to occlude the opening from the left atrium. The waist section is designed to self-center between the rims of the PFO.
– Other devices that have been developed include the Gore Cardioform Septal Occluder, STARFlex Septal Repair implant and the Lifetech Eccentric PFO Occluder. These function on similar principles to the above but with minor design changes to the implant.
Surgical Procedure for PFO Closure
The typical procedure for PFO closure involves the following steps:
– Computerized Tomography (CT) or transesophageal echocardiogram (TEE) is first performed to evaluate the PFO anatomy in detail.
– Under local anesthesia and light sedation, catheters are inserted through a vein in the groin and guided into position in the heart chambers under X-ray guidance.
– Through one catheter, a small wire guide is passed across the PFO from the right to left atrium.
– The collapsed PFO closure device is then advanced and delivered over the wire guide through the catheter across the PFO site.
– Once in proper position, the device self-expands to its pre-shaped form, with one disk on either side of the Patent Foramen Ovale (PFO) Closure Devices This closes the opening from the left atrium.
– The delivery cable and catheters are then gently removed, leaving the PFO closure device securely in place. The procedure takes about an hour in most cases.
Effectiveness of PFO Closure
Numerous randomized controlled trials and meta-analysis studies have been conducted to evaluate the effectiveness of PFO closure devices in reducing recurrent strokes or migraines. Some of the key findings have shown:
– Reduced risk of recurrent stroke – Trials like CLOSURE I found a 72% relative risk reduction in recurrent strokes with PFO closure compared to medical therapy alone over 2 years of follow up.
– Lower risk of migraine – The REDUCE trial found a 50% reduction in recurrent migraines at 18 months with PFO closure vs. sham procedure. Other trials reported 38-67% reductions.
– Long-term durability of closure – Long term follow up studies for over 5 years continued to show the PFO remained fully or partially closed in the majority of patients. Closure rates were up to 88% based on data from RESPECT trial.
– Improved quality of life – Several studies reported improvements in physical, social and role functioning as well as reduced headaches with PFO closure as measured by SF-36 surveys.
Risks & Complications
As with any cardiac procedure, there are risks involved with PFO closure. However, due to improvements in device design and implantation technique, risks have declined significantly. Major risks may include:
– Device embolization – Risk is 1% or less with most recent devices. May require surgical removal.
– Pericardial effusion – Tamponade requiring drainage occurs in less than 1% cases.
– Arrhythmias – <1% risk of new onset atrial fibrillation.
– Device fracture – Rare with self-centering devices but wire fracture reported. May not require intervention.
– Air embolism – Very rare risks requiring high flow oxygen support. Major risk reduction with improvements in catheter technology.
– Vascular complications – Groin hematoma or retroperitoneal bleed <2% risk due to large sheath catheter access.
Most complications can be managed without long term effects. Overall procedural risks are low, averaging less than 2% for serious adverse events. Safety continues improving annually.
In summary, PFO closure devices provide an effective and relatively low risk treatment option for reducing recurrent strokes and migraines in select patients with a patent foramen ovale. Multiple studies have demonstrated significant benefits with closure compared to medical therapy alone in lowering risks of further neurological events. While there are risks involved, advances in device design and implantation techniques have made the procedure very safe with few serious complications. For appropriate patients, PFO closure can potentially improve both longevity and quality of life outcomes.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it.